In Stock
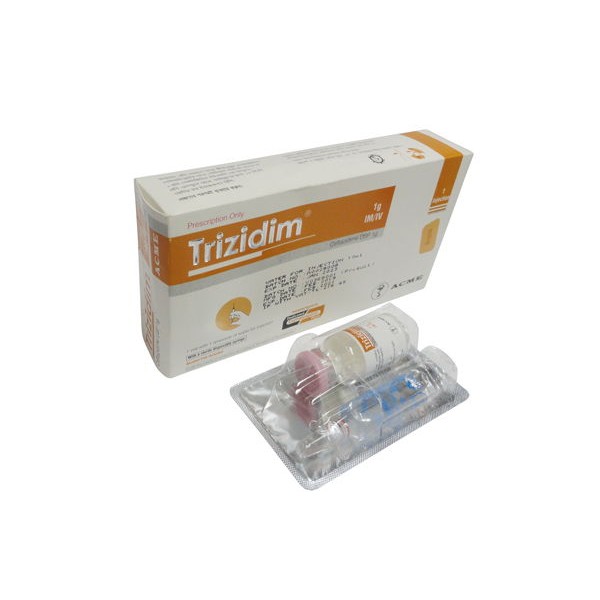
Trizidim 1g IM/IV Injection
Tk216
- Stock: In Stock
- Brand: Acme Laboratories Limited
- Product ID: Ceftazidime Pentahydrate
0 Pcs sold
3279 Interested
100% Secure Payment

This Item is for pre order
Estimated Delivery in
Description
Trizidim
(Ceftazidime) is a bactericidal cephalosporin antibiotic, which is resistant to most beta-lactamases and is active against a wide range of gram-positive and gramnegative bacteria. It is indicated for the treatment of single infections and for mixed infections caused by two or more susceptible organisms.
Composition
Trizidim 250 mg IM/IV : Each vial contains sterile Ceftazidime pentahydrate with sodium carbonate buffer equivalent to Ceftazidime USP 250 mg.
Trizidim 500 mg IM/IV : Each vial contains sterile Ceftazidime pentahydrate with sodium carbonate buffer equivalent to Ceftazidime USP 500 mg.
Trizidim 1 g IM/IV : Each vial contains sterile Ceftazidime pentahydrate with sodium carbonate buffer equivalent to Ceftazidime USP 1 g. Water for injection : Each ampoule contains 5 ml or 10 ml sterile water for injection BP for reconstitution.
Indications
Trizidim
is indicated for the treatment of the following
infections caused by susceptible organisms:
Lower respiratory tract infections
Skin & skin structure infections
Urinary tract infections
Bacterial septicemia
Bone & joint infections
Gynecological infections
Intra-abdominal infections
Central nervous system infections
Infections associated with haemo-and peritoneal dialysis and with continuous ambulatory peritoneal dialysis (CAPD)
Contraindications
Ceftazidime is contra-indicated in patients with known
hypersensitivity to Cephalosporins or any other ingredients of it.
Warnings
As with other beta-lactam antibiotics, before therapy with
Ceftazidime is instituted, careful inquiry should be made for a history of hypersensitivity reactions to Ceftazidime,
Cephalosporins, penicillins, or other drugs.
Side Effects
Clinical trial experience has shown that Ceftazidime is generally well tolerated. Adverse reactions are infrequent which may include:
Local: Phlebitis or thrombophlebitis with IV administration; pain and/or inflammation after IM injection. Gastrointestinal :
Diarrhoea, nausea, vomiting, abdominal pain, and very rarely oral thrush or colitis. Hypersensitivity : Urticarial rash, fever, pruritis, and very rarely angioedema and anaphylaxis
(bronchospasm and/or hypotension). Genito-urinary :
Candidiasis, vaginitis. Central nervous system : Headache,
dizziness and bad taste.
Precautions
There is no experimental evidence of embryopathic or teratogenic effects. Clinical experience with Ceftazidime has shown that this is not likely to be a problem at the recommended dose levels. There is no evidence that
Ceftazidime adversely affects renal function at normal therapeutic doses.
Use In Pregnancy & Lactation
Ceftazidime is of pregnancy category B. There is no experimental evidence of embryopathic or teratogenic effects attributable to ceftazidime; it should be administered with caution during the early month of pregnancy and early infancy. Nursing Mother: Ceftazidime is excreted in human milk in low concentrations. Caution should be exercised.
Drug Interactions
Nephrotoxicity has been reported following concomitant administration of Cephalosporins with aminoglycoside antibiotics or potent diuretics such as furosemide.
Storage condition
Unconstituted vials of Trizidim (Ceftazidime) Injection
should be stored at a temparature below 250C. Reconstituted
solutions are stable for up to 24 hours if stored between 20C and 80C and protected from light & moisture. Reconstituted solutions range from light yellow to amber color.
Supply
Trizidim (Ceftazidime) is supplied as sterile powder in glass vials.
Trizidim 250 mg IM/IV injection: Each box contains 1 vial of Ceftazidime USP 250 mg and one ampoule of 5 ml water for injection in blister pack, a 5 ml disposable syringe, a baby needle & an alcohol pad.
Trizidim 500 mg IM/IV injection: Each box contains 1 vial of Ceftazidime USP 500 mg and one ampoule of 5 ml water for injection in blister pack, a 5 ml disposable syringe, a baby needle & an alcohol pad.
Trizidim 1 g IM/IV injection: Each box contains 1 vial of Ceftazidime USP 1 g and one ampoule of 10 ml water for injection in blister pack, a 10 ml disposable syringe & an alcohol pad.






















%20Pvt.%20Ltd./Movicol-Oral-Powder-190x190.jpg)
