- Stock: In Stock
- Brand: Acme Laboratories Limited
- Product ID: Cefuroxime Axetil
100% Secure Payment

This Item is for pre order
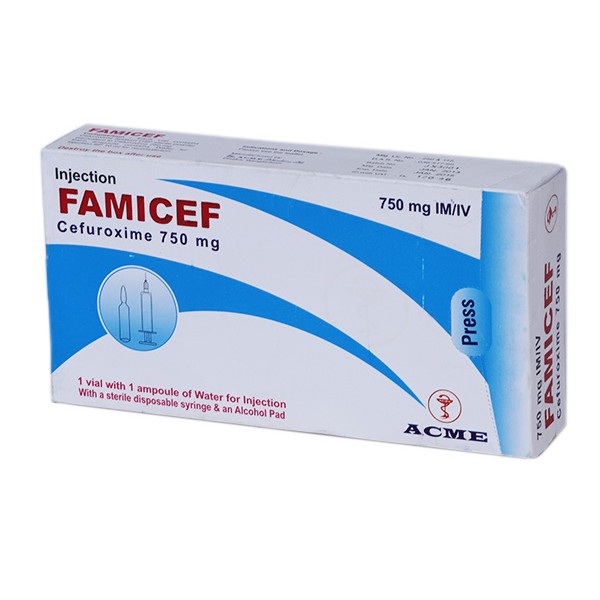
Famicef 750 IV Injection
Description
Famicef (Cefuroxime) is a well-characterized and effective antibacterial agent, which has broad-spectrum bactericidal activity against a wide range of common pathogens, including -lactamase producing strains.
Cefuroxime has good stability to bacterial -lactamase and consequently, is active against many Ampicillin-resistant and
Amoxicillin-resistant strains.
Composition
Famicef 250 Tablet : Each film-coated tablet contains Cefuroxime
Axetil USP equivalent to Cefuroxime 250 mg.
Famicef 500 Tablet : Each film-coated tablet contains Cefuroxime
Axetil USP equivalent to Cefuroxime 500 mg.
Famicef Powder for Suspension : After reconstitution, each 5 ml suspension contains Cefuroxime Axetil USP equivalent to Cefuroxime 125 mg.
Famicef DS Powder for Suspension : After reconstitution, each 5 ml suspension contains Cefuroxime Axetil USP equivalent to Cefuroxime 250 mg.
Indications
Famicef (Cefuroxime) is indicated in the treatment of
Upper respiratory tract infections: Ear, nose and throat infections such as otitis media, sinusitis, tonsillitis and pharyngitis.
Lower respiratory tract infection : Acute bronchitis, acute exacerbations of chronic bronchitis and pneumonia.
Skin and soft tissue infections : Furunculosis, pyoderma and impetigo.
Genito-urinary tract infections: Pyelonephritis, urethritis and cystitis.
Gonorrhoea: Acute uncomplicated gonococcal urethritis and cervicitis.
Early Lyme Disease and subsequent prevention of late Lyme Disease
Contraindications
Famicef (Cefuroxime) is contraindicated in patients with known hypersensitivity to Cephalosporins or any other components of the drug.
Side effects
Famicef (Cefuroxime) is generally well tolerated. Cefuroxime has been associated with nausea and vomiting in a small number of patients.
Precautions
As with other antibiotics, prolonged use may result in the over growth of non-susceptible organisms (e.g. Candida, Enterococci, Clostridium difficile), which may require interruption of treatment. Precaution should be taken patient with renal impairment.
Use in pregnancy & lactation
Famicef (Cefuroxime) is of pregnancy Category B. It can be used in the later period of pregnancy. Cefuroxime is excreted in breast milk, and consequently caution should be exercised when Cefuroxime is administered to a nursing mother.
Drug interaction
No potentially hazardous interactions have been reported.
Supply
Famicef 250 Tablet : Each box contains 5 4's tablets in Alu-Alu blister strip.
Famicef 500 Tablet : Each box contains 2 4's tablets in Alu-Alu blister strip.
Famicef Powder for Suspension : Each bottle contains dry powder to reconstitute 70 ml suspension.
Famicef DS Powder for Suspension: Each bottle contains dry powder to reconstitute 35 ml suspension.


























%20Pvt.%20Ltd./Movicol-Oral-Powder-190x190.jpg)
