- Stock: In Stock
- Brand: Acme Laboratories Limited
- Product ID: Azilsartan Medoxomil
100% Secure Payment

This Item is for pre order
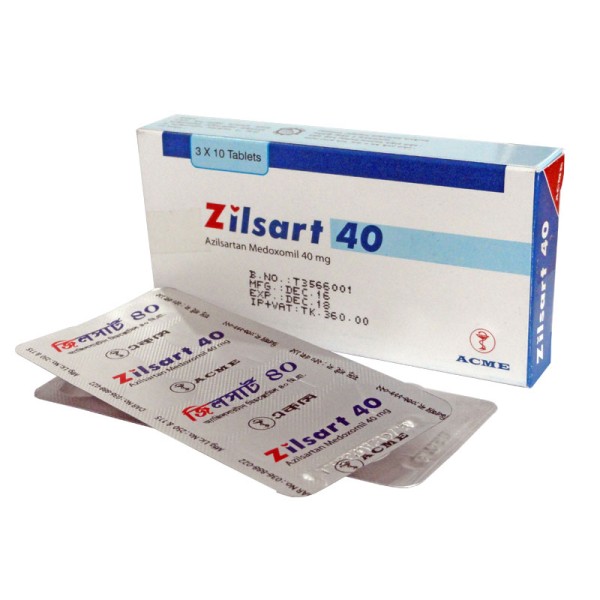
Zilsart 40 mg Tab
Description
Zilsart (Azilsartan Medoxomil), a prodrug, is hydrolyzed to Azilsartan during absorption from the gastrointestinal tract. Zilsart (Azilsartan
Medoxomil) is a selective AT1 sub-type angiotensin II receptor antagonist. Zilsart (Azilsartan Medoxomil) blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in vascular smooth muscle and thereby decreases blood pressure.
Composition
Zilsart 40 : Each film-coated tablet contains Azilsartan Kamedoxomil
INN equivalent to Azilsartan Medoxomil 40 mg.
Indication
Zilsart (Azilsartan Medoxomil) is indicated for the treatment of hypertension. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. It may be used alone or in combination with other antihypertensive agents.
Dosage & Administration
Adults
The recommended starting dose of Zilsart is 40 mg once daily. The dose may be increased to a maximum of 80 mg once daily for patients whose blood pressure is not adequately controlled at the lower dose.
Zilsart may be administrated with other antihypertensive agents for
further blood pressure reduction if needed. Azilsartan may be taken with or without food.
Renal Impairment
No initial dose adjustment is required with mild-to-severe renal impairment or end stage renal disease. Patients with moderate to severe renal impairment are more likely to report abnormally high serum creatinine values.
Hepatic Impairment
Dose adjustment is not necessary with mild-to-moderate hepatic impairment. Azilsartan has not been studied in patients with severe hepatic impairment and therefore its use is not recommended in this patient group.
Elderly patients
No adjustment with Azilsartan is necessary in elderly patients.
Side Effects
The most common adverse reaction in adults was diarrhea, asthenia, fatigue, muscle spasm, dizziness, cough etc.
Contraindication
Hypersensitivity to Azilsartan or any component of the formulation.
Precaution
Hypotension in volume or salt-depleted patients, symptomatic hypotension may occur after initiation of treatment with Azilsartan.
Impaired renal function, as a consequence of inhibiting the reninangiotensin system, changes in renal function may be anticipated in susceptible individuals treated with Azilsartan.
Use in Pregnancy & Lactation
Pregnancy: Pregnancy Category D
Azilsartan should not be used in pregnancy and it should be discontinued as soon as possible when pregnancy is detected.
Lactation: It should not be used during lactation, as there is no information in human on the excretion of Azilsartan in breast milk.
Because of the potential for adverse effects on the nursing infants, a decision should be made whether to discontinue the nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Drug Interactions
No significant drug interactions have been observed in studies of
Azilsartan medoxomil given with amlodipine, antacids, chlorthalidone, digoxin, fluconazole, glyburide, ketoconazole, metformin, pioglitazone and warfarin. Therefore, Azilsartan may be used concomitantly with these medications. In patients who are elderly, volume-depleted or who have compromised renal function, co-administration of NSAIDs, with
Azilsartan may result in deterioration of renal function. The antihypertensive effect of Azilsartan may be attenuated by NSAIDs.
Over dose
Limited data are available related to overdose in human. During controlled clinical trials in healthy subjects, once daily dose up to 320 mg of Azilsartan were administered for 7 days and were well tolerated.
Supply
Zilsart 40: Each box contains 3x10 tablets in Alu-Alu blister pack.



























%20Pvt.%20Ltd./Movicol-Oral-Powder-190x190.jpg)
